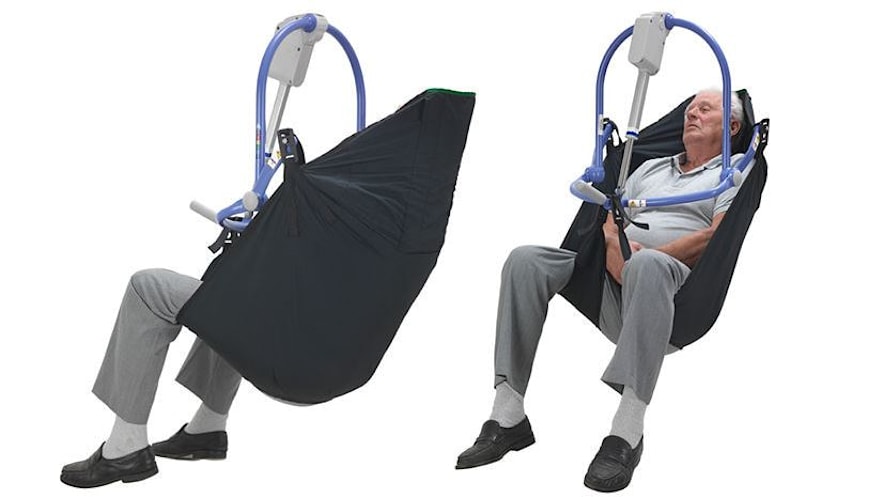
The Therapeutic Goods Administration (TGA) issued the warning after an investigation into the All Day Sling made by medical device manufacturer, Arjo Australia, showed the apparatus could cause bed sores and welts.
The sling has only been approved for temporary use to lift or transfer residents, and not for all-day use (despite its name). The TGA was prompted to investigate following concerns that the device was being kept in use for several hours in some patients’ cases, increasing the risk of pressure sores.
One man, Ron van Leer, told The Guardian that he complained to the TGA about the sling after he realised it was being used for four to eight hours at a time on his 92-year-old mother’s bed.
He has also made a submission to the Royal Commission about the sling.